Using a nucleic acid-based therapy to target genetic mutations, eye researchers at the University of Wisconsin–Madison and University of Iowa will develop and test a new therapy for restoring vision in children and adults with severe visual impairment or blindness due to inherited retinal disorders.
The new project, titled “Restoring Vision with High-Fidelity Nonsense Codon Correction”, is a collaboration between UW-Madison’s School of Medicine and Public Health and College of Engineering, the Wisconsin Institute for Discovery (WID) at UW-Madison, and the University of Iowa (UI). It is funded by a five-year, $7.7 million award from the National Institutes of Health/National Eye Institute (NIH/NEI) as part of the NEI Translational Research Program to Develop Novel Therapies and Devices for the Treatment of Visual System Disorders (R24).
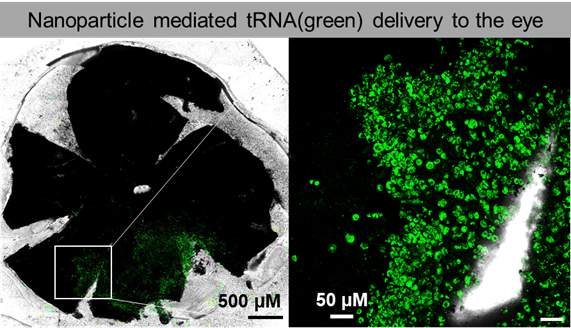
The project, led by UW researchers Bikash Pattnaik, PhD, assistant professor of pediatrics and ophthalmology and visual sciences, Shaoqin “Sarah” Gong, PhD, professor of biomedical engineering at WID, David Gamm, MD, PhD, professor of ophthalmology and visual sciences and director of the McPherson Eye Research Institute, and UI researcher and UW alumnus Christopher Ahern, PhD, professor of molecular physiology and biophysics at the Iowa Neuroscience Institute, will develop a nucleic acid-based therapy using anticodon edited transfer RNA (ace-tRNA) to target nonsense mutations that cause blindness. A nonsense mutation is a point mutation in the DNA sequence that deleteriously stops healthy protein synthesis prematurely. The disease phenotype is often severe, which, if left untreated, results in blindness or severe visual impairment that is likely to become permanent blindness later in life.

“For pediatric populations, this new therapy will be tested to treat inherited retinal disorders like Leber congenital amaurosis, Best disease, or more frequent congenital stationary night blindness,” stated Pattnaik. “Targeting a therapy early on for most genetic disease is extremely important, but this therapy could be used for people of all ages based on clinical evidence.”
Currently, there are no FDA-approved treatments and only a limited number of therapies for nonsense mutations being tested in clinical trials in humans. This novel project aims to advance ace-tRNA technology toward clinical trials for a wide range of genetic diseases that cause blindness.
The Future of Ophthalmology // Drs. Bikash Pattnaik and David Gamm
This story was originally published by the UW Department of Pediatrics in the Milestones February 2021 department newsletter.